
Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18(l) + 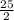 O2(g) → 8CO2(g) + 9H2O(g) ΔH∘rxn = −5074.1 kJ
What mass of octane (in g) is required to produce 8210 kJ of heat?
O2(g) → 8CO2(g) + 9H2O(g) ΔH∘rxn = −5074.1 kJ
What mass of octane (in g) is required to produce 8210 kJ of heat?
Express your answer to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
Octane (C8H18) is a component of gasoline that burns according to the following equation: C8H18(l) +...
Questions



History, 03.12.2020 01:00


Mathematics, 03.12.2020 01:00

Chemistry, 03.12.2020 01:00


English, 03.12.2020 01:00





Mathematics, 03.12.2020 01:00

Computers and Technology, 03.12.2020 01:00

Geography, 03.12.2020 01:00


Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

Computers and Technology, 03.12.2020 01:00



