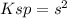Chemistry, 13.07.2021 15:10 smithmariah7426
In order to promote the common ion effect, the concentration of the common ion must first:
a. increase
b. decrease
c. be equal to its equilibrium value
d. depends on the equilibrium

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
In order to promote the common ion effect, the concentration of the common ion must first:
a. incr...
Questions

Mathematics, 12.10.2020 22:01




History, 12.10.2020 22:01


History, 12.10.2020 22:01

History, 12.10.2020 22:01

Biology, 12.10.2020 22:01



History, 12.10.2020 22:01


Mathematics, 12.10.2020 22:01




English, 12.10.2020 22:01

Computers and Technology, 12.10.2020 22:01

![Ksp=[Ag^+][Cl^-]](/tpl/images/1393/2378/21897.png)