
Chemistry, 13.07.2021 16:30 aesthetickait
The following pairs of soluble solutions can be mixed. In some cases, this leads to the formation of an insoluble precipitate. Decide, in each case, whether or not an insoluble precipitate is formed.
a. K2S and NH4Cl
b. CaCl2 and NH4CO3
c. Li2S and MnBr2
d. Ba(NO3)2 and Ag2SO4
e. RbCO3 and NaCl

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
The following pairs of soluble solutions can be mixed. In some cases, this leads to the formation of...
Questions


Chemistry, 05.05.2020 03:30



Mathematics, 05.05.2020 03:30



English, 05.05.2020 03:30



History, 05.05.2020 03:30

World Languages, 05.05.2020 03:30

Social Studies, 05.05.2020 03:30



English, 05.05.2020 03:31



History, 05.05.2020 03:31

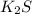 and
and  :
: and
and  :
: forms.
forms. and
and 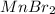 :
: forms.
forms.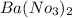 and
and  :
:  and
and  :
: ⇒ soluble.
⇒ soluble. ⇒ soluble,
⇒ soluble, 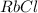 ⇒ soluble,
⇒ soluble,  ⇒ soluble.
⇒ soluble.

