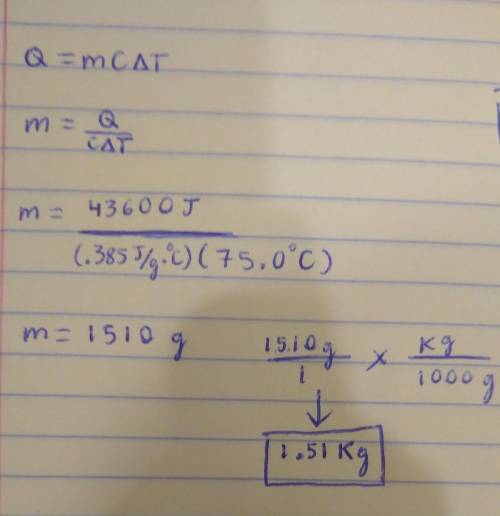Chemistry, 13.07.2021 17:00 darkghostmist
A sample of Copper absorbs 43.6 KJ of heat, resulting in a temperature rise of 75.0 oC, determine the mass (in Kg) of the copper sample, if the specific heat capacity of Copper is 0.385 J/g oC

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
A sample of Copper absorbs 43.6 KJ of heat, resulting in a temperature rise of 75.0 oC, determine th...
Questions


Mathematics, 08.04.2020 12:50





Geography, 08.04.2020 12:51

Geography, 08.04.2020 12:51

English, 08.04.2020 12:51

Mathematics, 08.04.2020 12:51

Mathematics, 08.04.2020 12:51


Mathematics, 08.04.2020 12:52

Mathematics, 08.04.2020 12:52

Mathematics, 08.04.2020 12:52

Mathematics, 08.04.2020 12:53

Mathematics, 08.04.2020 12:53