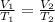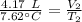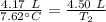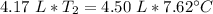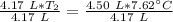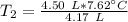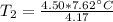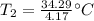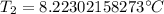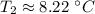Chemistry, 14.07.2021 04:20 Sparkledog
A 4.17 L volume of oxygen gas measured at 7.62 °C is expanded to a new volume of 4.50 L. Calculate the temperature (in oC) of the gas at the higher volume, assuming no change in pressure.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
A 4.17 L volume of oxygen gas measured at 7.62 °C is expanded to a new volume of 4.50 L. Calculate t...
Questions

Mathematics, 24.09.2019 14:30



Arts, 24.09.2019 14:30


Mathematics, 24.09.2019 14:30







Geography, 24.09.2019 14:30





English, 24.09.2019 14:30

Mathematics, 24.09.2019 14:30