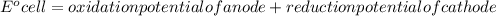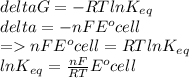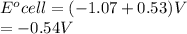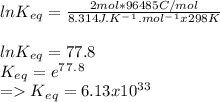Chemistry, 14.07.2021 20:10 SkyeShadow525
Determine the equilibrium constant, Keq, at 25°C for the reaction
2Br- (aq) + I2(s) <--> Br2(l) + 2I- (aq)
Eocell = (0.0257/n) lnKeq, Calculate Eocell from Use this equation to calculate K value.
Eo (I2/I-) = +0.53, Eo (Br2/Br-) = +1.07,

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
Determine the equilibrium constant, Keq, at 25°C for the reaction
2Br- (aq) + I2(s) <--> Br2(...
Questions



Mathematics, 02.07.2019 23:30



Mathematics, 02.07.2019 23:30

Mathematics, 02.07.2019 23:30



Mathematics, 02.07.2019 23:30








Advanced Placement (AP), 02.07.2019 23:30