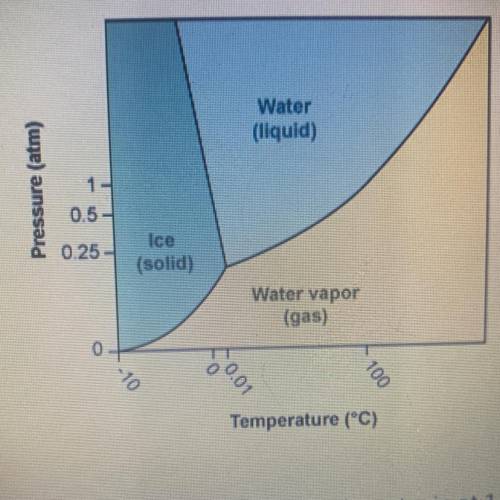
Using the phase diagram for H20, what phase is water in at 1 atm pressure
and -5°C?
A. It is...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Questions

English, 08.05.2022 02:50

Mathematics, 08.05.2022 02:50

Business, 08.05.2022 02:50

Mathematics, 08.05.2022 03:00


Mathematics, 08.05.2022 03:00

Biology, 08.05.2022 03:10

English, 08.05.2022 03:10



Biology, 08.05.2022 03:20


Mathematics, 08.05.2022 03:40


Business, 08.05.2022 03:40