Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
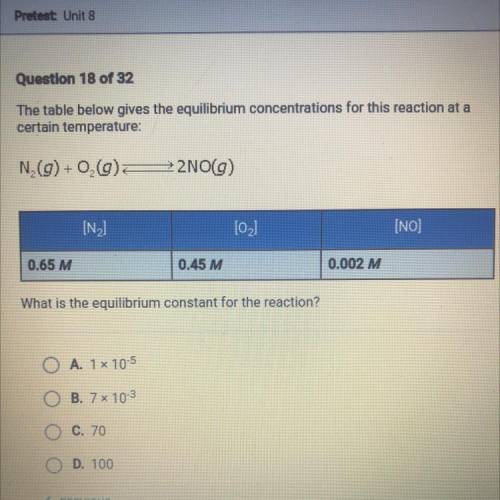
The table below gives the equilibrium concentrations for this reaction at a certain temperature : N2...
Questions





Mathematics, 16.06.2021 18:30

Mathematics, 16.06.2021 18:30

Computers and Technology, 16.06.2021 18:30








Mathematics, 16.06.2021 18:30

Mathematics, 16.06.2021 18:30




English, 16.06.2021 18:30