
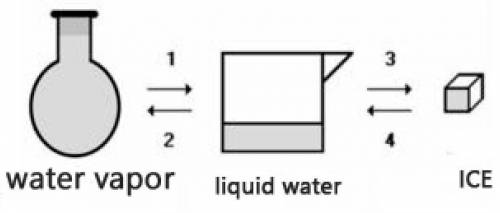
Consider the transformations a water sample undergoes without external pressure variation
(a) Transformations 2 and 4 are endothermic.
(b) Transformations 1 and 2 are exothermic.
(c) The amount of energy absorbed in 3 is equal to the amount released in 1.
(d) The amount of energy released in 2 is equal to the amount released in 4.
(e) The change of physical state does not involve heat energy.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Consider the transformations a water sample undergoes without external pressure variation
(a) Trans...
Questions

Mathematics, 29.01.2021 20:40

Arts, 29.01.2021 20:40



Chemistry, 29.01.2021 20:40

Arts, 29.01.2021 20:40

Mathematics, 29.01.2021 20:40

Mathematics, 29.01.2021 20:40


Chemistry, 29.01.2021 20:40


Physics, 29.01.2021 20:40



Mathematics, 29.01.2021 20:40





English, 29.01.2021 20:40



