
In aqueous solution the Hg2+ ion forms a complex with four iodide anions. Write the formation constant expression for the equilibrium between the hydrated metal ion and the aqueous complex. Under that, write the balanced chemical equation for the first step in the formation of the complex.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

You know the right answer?
In aqueous solution the Hg2+ ion forms a complex with four iodide anions. Write the formation consta...
Questions






Social Studies, 24.06.2019 11:30

History, 24.06.2019 11:30



Biology, 24.06.2019 11:30

Biology, 24.06.2019 11:30

Mathematics, 24.06.2019 11:30

English, 24.06.2019 11:30

Social Studies, 24.06.2019 11:30

Mathematics, 24.06.2019 11:30





Mathematics, 24.06.2019 11:30

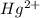 ion is used to represent a
ion is used to represent a ![[Hg(H_2O)_4]^{2+}](/tpl/images/1394/5126/65dfb.png) .
.
![[Hgl_4]^{2-}](/tpl/images/1394/5126/970b6.png) .
. ![[Hg(H_2O)_4]^{2+} +4I^{-} \to [HgI_4]^{2-}+4H_20](/tpl/images/1394/5126/c93e6.png)
![k_f=\frac{[HgI_4]^{2-}}{[Hg(H_2O)_4]^{2+} [I^{-}]^4}](/tpl/images/1394/5126/9ffc5.png)
![[Hg(H_2O)_4]^{2+} +I^{-} \to [HgI(H_2O)_3]^{+}+H_20](/tpl/images/1394/5126/c074d.png)


