
Chemistry, 15.07.2021 18:40 Aurionna101
How would the equilibrium change if the temperature were increased in SO 2 (g)+NO 2 (g) NO(g)+SO 3 (g)+heat ?
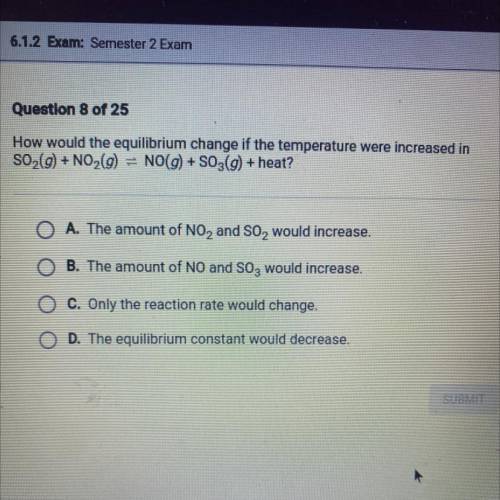

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
How would the equilibrium change if the temperature were increased in SO 2 (g)+NO 2 (g) NO(g)+SO 3 (...
Questions




History, 16.10.2019 07:30

Mathematics, 16.10.2019 07:30

Chemistry, 16.10.2019 07:30




Social Studies, 16.10.2019 07:30





History, 16.10.2019 07:30


English, 16.10.2019 07:30

English, 16.10.2019 07:30

Business, 16.10.2019 07:30

Mathematics, 16.10.2019 07:30



