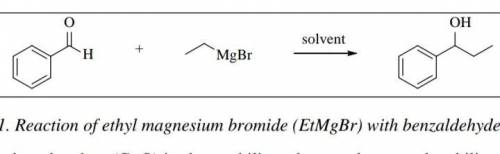
Chemistry, 15.07.2021 22:30 moneymaleia9264
If 0.650 mL of benzaldehyde reacts with enough of the Grignard reagent, calculate the theoretical yield (in grams) of the alcohol product. Show calculation with units for full credit.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2

You know the right answer?
If 0.650 mL of benzaldehyde reacts with enough of the Grignard reagent, calculate the theoretical yi...
Questions


History, 06.05.2020 06:02

Mathematics, 06.05.2020 06:02

Health, 06.05.2020 06:02

English, 06.05.2020 06:02


Mathematics, 06.05.2020 06:02












History, 06.05.2020 06:02

Mathematics, 06.05.2020 06:03