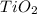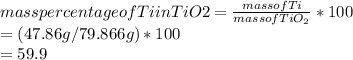A sample of rutile, an ore of titanium consisting principally of TiO2(s), was found to be 65.2% TiO2(s) by mass, with the remainder being sand impurities. what is the minimum number of metric tons of the ore that must be processed to obtain 10.0 metric tons of titanium

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
A sample of rutile, an ore of titanium consisting principally of TiO2(s), was found to be 65.2% TiO2...
Questions



Biology, 25.08.2019 00:20

Computers and Technology, 25.08.2019 00:20


Biology, 25.08.2019 00:20

Computers and Technology, 25.08.2019 00:20

English, 25.08.2019 00:20



Social Studies, 25.08.2019 00:20






Arts, 25.08.2019 00:30

Biology, 25.08.2019 00:30

Mathematics, 25.08.2019 00:30