
Chemistry, 16.07.2021 03:40 abbypoletick
The reversible reaction: 2SO2(g) O2(g) darrow-tn. gif 2SO3(g) has come to equilibrium in a vessel of specific volume at a given temperature. Before the reaction began, the concentrations of the reactants were 0.060 mol/L of SO2 and 0.050 mol/L of O2. After equilibrium is reached, the concentration of SO3 is 0.040 mol/L. What is the equilibrium concentration of O2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
The reversible reaction: 2SO2(g) O2(g) darrow-tn. gif 2SO3(g) has come to equilibrium in a vessel of...
Questions




Mathematics, 15.05.2021 04:10



Chemistry, 15.05.2021 04:10


English, 15.05.2021 04:10

Mathematics, 15.05.2021 04:10

History, 15.05.2021 04:10


Mathematics, 15.05.2021 04:10

Mathematics, 15.05.2021 04:10

Mathematics, 15.05.2021 04:10


Arts, 15.05.2021 04:10



Mathematics, 15.05.2021 04:10

![[O_2]_{eq}=0.030M](/tpl/images/1395/0026/cfd77.png)
![[O_2]_{eq}=0.050M-x](/tpl/images/1395/0026/a8660.png)
 can be found considering the equilibrium of SO3:
can be found considering the equilibrium of SO3:![[SO_3]_{eq}=2x=0.040M](/tpl/images/1395/0026/c4625.png)
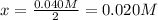
![[O_2]_{eq}=0.050M-0.020M=0.030M](/tpl/images/1395/0026/b7171.png)


