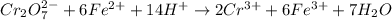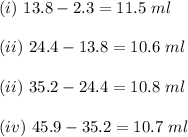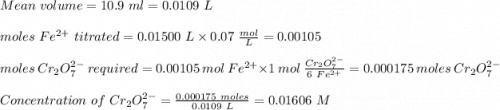Chemistry, 16.07.2021 20:00 jcazaresroman7308
ProblemWhat is the concentration of a tin(ll) chloride solution prepared from a sample of tin ore?Experimental DesignThe potassium dichromate solution is first standardized by titration with 15.00 mL of an acidified 0.07 mol/L solution of the primary standard, iron(II) ammonium sulfate-6-water. The standardized dichromate solution is then titrated against a sample of the acidified tin(II) chloride solution (You will do this step in the next question). Evidence TITRATION OF IRON(lI) SOLUTION(volume of K2Cr2O7(aq) required to react with 15.00 ml of 0.07 mol/L Fe2+(aq)) Trial1234Final buretreading(ml) 13.824.435.245.9Initial buretreading (ml) 2.313.824.435.2Find the concentration of the Cr2O72-(aq) in mol/L: (give your answer to 4 decimal places)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
ProblemWhat is the concentration of a tin(ll) chloride solution prepared from a sample of tin ore?Ex...
Questions




Mathematics, 17.05.2021 16:30



Mathematics, 17.05.2021 16:30




Mathematics, 17.05.2021 16:30




Mathematics, 17.05.2021 16:30




Computers and Technology, 17.05.2021 16:30