
Chemistry, 16.07.2021 23:30 Mayjay2827
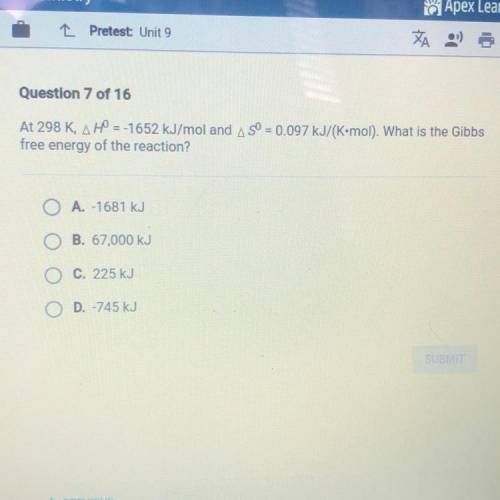
At 298 K, H = -1652 kJ/mol and S = 0.097 kJ/(K•mol). What is the Gibbs free energy of the reaction?
A.-1681 kJ
B. 67,000 kJ
C. 225 kJ
D. -745 kJ

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
At 298 K, H = -1652 kJ/mol and S = 0.097 kJ/(K•mol). What is the Gibbs free energy of the reaction?...
Questions

Mathematics, 11.02.2021 16:00

Mathematics, 11.02.2021 16:00

English, 11.02.2021 16:00

Mathematics, 11.02.2021 16:00

Biology, 11.02.2021 16:00


Mathematics, 11.02.2021 16:00

Mathematics, 11.02.2021 16:00


Mathematics, 11.02.2021 16:00



Mathematics, 11.02.2021 16:00

Computers and Technology, 11.02.2021 16:00

History, 11.02.2021 16:00

Spanish, 11.02.2021 16:00

English, 11.02.2021 16:00


French, 11.02.2021 16:00



