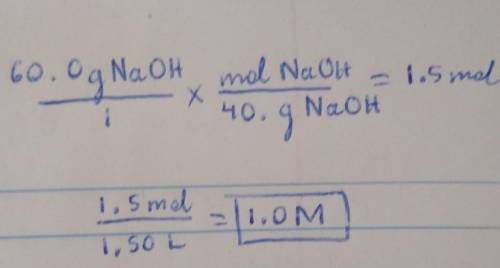Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1
You know the right answer?
Calculate the molarity of a solution consisting of 60.0 g of NaOH in 1.50 L of solution....
Questions



Mathematics, 28.08.2020 04:01

Mathematics, 28.08.2020 04:01



Mathematics, 28.08.2020 04:01

Mathematics, 28.08.2020 04:01

Health, 28.08.2020 04:01

Mathematics, 28.08.2020 04:01


Mathematics, 28.08.2020 04:01

History, 28.08.2020 04:01

Physics, 28.08.2020 04:01


History, 28.08.2020 04:01

Mathematics, 28.08.2020 04:01

Mathematics, 28.08.2020 04:01

Spanish, 28.08.2020 04:01

Mathematics, 28.08.2020 04:01