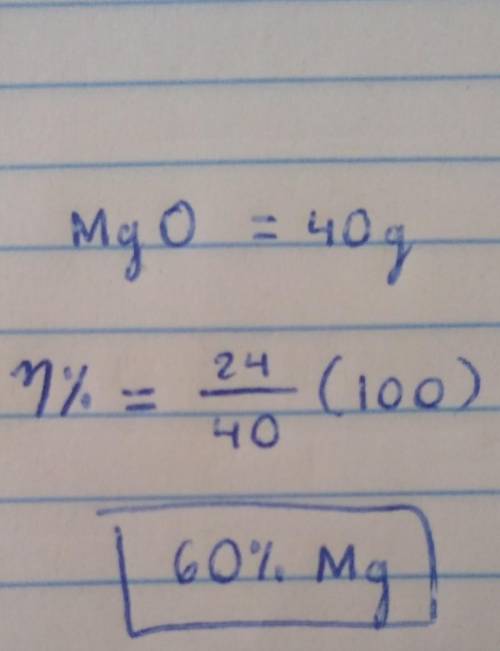Chemistry, 17.07.2021 01:00 rachelreed
1a. calculate the relative formula mass of magnesium oxide when the relative atomic masses are O=16 Mg=24 1b. calculate the percentage by mass of magnesium in magnesium oxide

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
1a. calculate the relative formula mass of magnesium oxide when the relative atomic masses are O=16...
Questions



Social Studies, 06.01.2021 21:10

Mathematics, 06.01.2021 21:10



Mathematics, 06.01.2021 21:10

Chemistry, 06.01.2021 21:10

Mathematics, 06.01.2021 21:10

Arts, 06.01.2021 21:10

History, 06.01.2021 21:10

Mathematics, 06.01.2021 21:10



History, 06.01.2021 21:10

Social Studies, 06.01.2021 21:10



Health, 06.01.2021 21:10

History, 06.01.2021 21:10