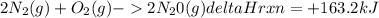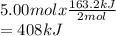Chemistry, 17.07.2021 08:40 amarshall90
Consider the reaction below. How much heat is absorbed if 5.00 moles of nitrogen react
with excess oxygen?
2 N2 (8) + O2(g) → 2 N20 (8) AHrxn- +163.2 kJ

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3
You know the right answer?
Consider the reaction below. How much heat is absorbed if 5.00 moles of nitrogen react
with excess...
Questions




English, 19.01.2020 00:31

Mathematics, 19.01.2020 00:31

Mathematics, 19.01.2020 00:31






History, 19.01.2020 00:31


Mathematics, 19.01.2020 00:31

Social Studies, 19.01.2020 00:31



Mathematics, 19.01.2020 00:31


Chemistry, 19.01.2020 00:31