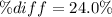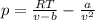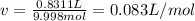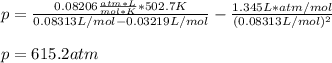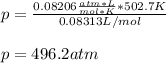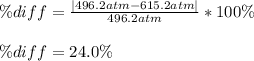Chemistry, 18.07.2021 03:50 andrea1704
According to the ideal gas law, a 9.998 mol sample of argon gas in a 0.8311 L container at 502.7 K should exert a pressure of 496.2
atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For Ar
gas, a = 1.345 L’atm/mol? and b = 3.219x10-2 L/mol.
Pideal – Puan der Waals |
Percent difference
x 100

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
According to the ideal gas law, a 9.998 mol sample of argon gas in a 0.8311 L container at 502.7 K s...
Questions


Computers and Technology, 21.09.2020 09:01

Mathematics, 21.09.2020 09:01



English, 21.09.2020 09:01



Mathematics, 21.09.2020 09:01


Spanish, 21.09.2020 09:01

Mathematics, 21.09.2020 09:01







Mathematics, 21.09.2020 09:01

Biology, 21.09.2020 09:01