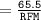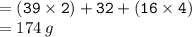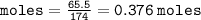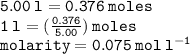Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3


Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
Calculate the molarity of a solution consisting of 65.5 g of K2S0 4 in 5.00 L of solution. ...
Questions

Social Studies, 09.07.2019 14:30


Mathematics, 09.07.2019 14:30

Biology, 09.07.2019 14:30

Arts, 09.07.2019 14:30


Mathematics, 09.07.2019 14:30



Arts, 09.07.2019 14:30

Chemistry, 09.07.2019 14:30

Mathematics, 09.07.2019 14:30

Arts, 09.07.2019 14:30

Mathematics, 09.07.2019 14:30

English, 09.07.2019 14:30

Mathematics, 09.07.2019 14:30

Biology, 09.07.2019 14:30

Arts, 09.07.2019 14:30