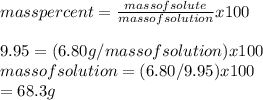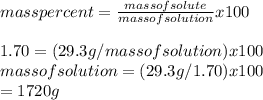Chemistry, 19.07.2021 17:30 whocares1234
Calculate the amount of water (in grams) that must be added to (a) 6.80 g of urea [(NH2)2CO] in the preparation of a 9.95 percent by mass solution: g (b) 29.3 g of MgBr2 in the preparation of a 1.70 percent mass solution: g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Calculate the amount of water (in grams) that must be added to (a) 6.80 g of urea [(NH2)2CO] in the...
Questions

Mathematics, 07.12.2021 19:30

English, 07.12.2021 19:30


Mathematics, 07.12.2021 19:30





Computers and Technology, 07.12.2021 19:30


English, 07.12.2021 19:40

History, 07.12.2021 19:40


English, 07.12.2021 19:40



World Languages, 07.12.2021 19:40

Mathematics, 07.12.2021 19:40