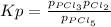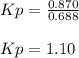A reaction vessel is charged with phosphorus pentachloride, which partially decomposes to phosphorus trichloride and molecular chlorine according to the following reaction:
PCl5(g)â PCl3(g)+Cl2(g)
When the system comes to equilibrium at 250.0°C, the equilibrium partial pressures are: PPCl5 = 0.688 atm and PPCl3 = PCl2 = 0.870 atm.
Required:
What is the value of Kp at this temperature?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
A reaction vessel is charged with phosphorus pentachloride, which partially decomposes to phosphorus...
Questions

Health, 21.06.2019 20:00

Spanish, 21.06.2019 20:00

Spanish, 21.06.2019 20:00

Mathematics, 21.06.2019 20:00


Advanced Placement (AP), 21.06.2019 20:00


Spanish, 21.06.2019 20:00



Spanish, 21.06.2019 20:00

World Languages, 21.06.2019 20:00

Mathematics, 21.06.2019 20:00






Mathematics, 21.06.2019 20:00