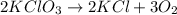Chemistry, 20.07.2021 01:00 mimithurmond03
When a mixture of potassium chlorate and an appropriate catalyst is heated in an open test tube, potassium chloride and oxygen gas are produced. This reaction is not reversible because a the reaction does not go to completion b the system reaches equilibrium c the system is closed d the oxygen escapes

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
When a mixture of potassium chlorate and an appropriate catalyst is heated in an open test tube, pot...
Questions



Mathematics, 23.10.2020 19:10

Biology, 23.10.2020 19:10





Mathematics, 23.10.2020 19:10

History, 23.10.2020 19:10

Law, 23.10.2020 19:10

Computers and Technology, 23.10.2020 19:10

Spanish, 23.10.2020 19:10

Mathematics, 23.10.2020 19:10

Biology, 23.10.2020 19:10




Mathematics, 23.10.2020 19:10

Mathematics, 23.10.2020 19:10