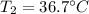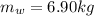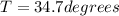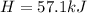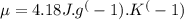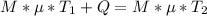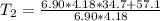Chemistry, 20.07.2021 04:10 hayden5928
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 6.90kg of water at 34.7 degrees C . During the reaction 57.1kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J. g^(-1).K^(-1) . Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 6....
Questions




Mathematics, 27.08.2020 02:01




Business, 27.08.2020 02:01

Spanish, 27.08.2020 02:01

Mathematics, 27.08.2020 02:01


Mathematics, 27.08.2020 02:01

Mathematics, 27.08.2020 02:01


History, 27.08.2020 02:01

Law, 27.08.2020 02:01

Mathematics, 27.08.2020 02:01



English, 27.08.2020 02:01