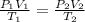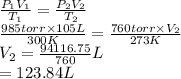Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
A sample of neon gas occupies 105 L at 27°C under a pressure of
985 torr. What volume would it occu...
Questions

Computers and Technology, 12.03.2021 06:10

Mathematics, 12.03.2021 06:10



Mathematics, 12.03.2021 06:10


Chemistry, 12.03.2021 06:10

Health, 12.03.2021 06:10



Mathematics, 12.03.2021 06:10



Mathematics, 12.03.2021 06:10




Physics, 12.03.2021 06:10

Mathematics, 12.03.2021 06:10

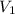 = 105 L,
= 105 L, 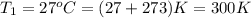 ,
, 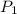 = 985 torr
= 985 torr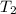 = 273 K,
= 273 K, 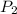 = 760 K,
= 760 K, 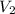 = ?
= ?