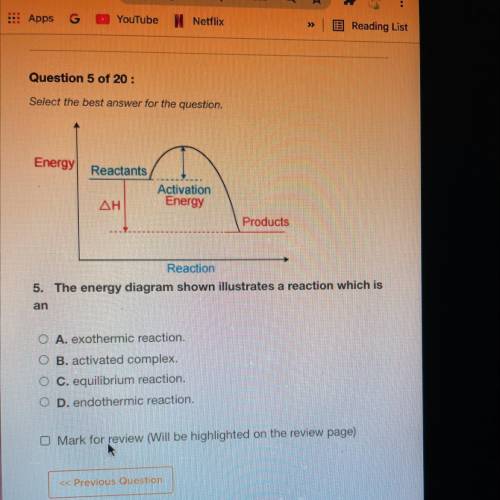
5. The energy diagram shown illustrates a reaction which is
an
A. exothermic reaction.
...

Chemistry, 20.07.2021 08:30 leewalker1341
5. The energy diagram shown illustrates a reaction which is
an
A. exothermic reaction.
B. activated complex.
C. equilibrium reaction.
D. endothermic reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Questions

Mathematics, 19.10.2019 15:50


Chemistry, 19.10.2019 15:50

Mathematics, 19.10.2019 15:50




Mathematics, 19.10.2019 15:50

Physics, 19.10.2019 15:50

Biology, 19.10.2019 15:50


Mathematics, 19.10.2019 15:50

Geography, 19.10.2019 15:50

Arts, 19.10.2019 15:50


Mathematics, 19.10.2019 15:50

Social Studies, 19.10.2019 15:50


Mathematics, 19.10.2019 15:50



