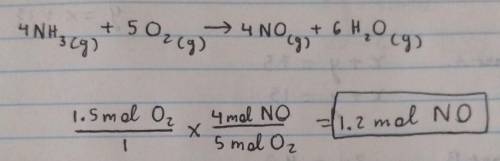Gaseous ammonia chemically reacts with oxygen gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of nitrogen monoxide produced by the reaction of 1.5 moles of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1


Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
Gaseous ammonia chemically reacts with oxygen gas to produce nitrogen monoxide gas and water vapor....
Questions

Mathematics, 19.02.2021 19:10

Mathematics, 19.02.2021 19:10


Mathematics, 19.02.2021 19:10


English, 19.02.2021 19:10










Physics, 19.02.2021 19:10




Mathematics, 19.02.2021 19:10