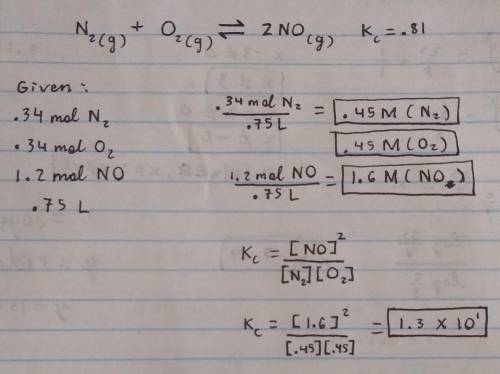Chemistry, 21.07.2021 01:20 Goodgirlkl12k
The decomposition of nitric oxide to molecular nitrogen and oxygen occurs at high temperatures according to the reaction:
N2(g)+O2(g)⟺2NO(g)Kc=0.81
At the start of the reaction, 0.34 mol N2, 0.34 mol O2 and 1.2 mol NO are introduced into a 0.75 L reaction chamber and allowed to equilibrate.
Required:
What is the concentration of NO (in M) at equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1


Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
The decomposition of nitric oxide to molecular nitrogen and oxygen occurs at high temperatures accor...
Questions