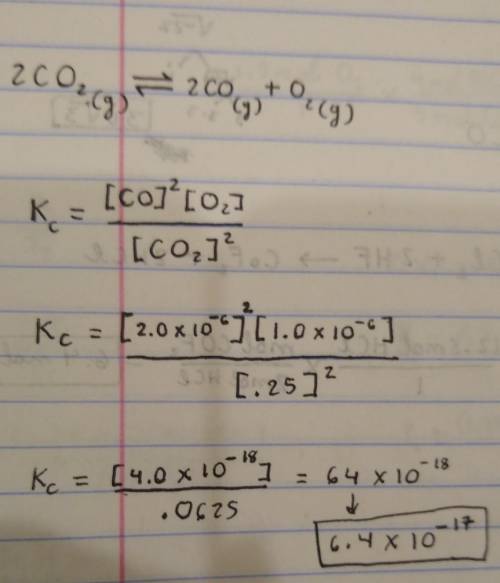Chemistry, 21.07.2021 06:10 gabrielgbobo99
Calculate K for 2CO2(g) ⇔ 2CO(g) + O2(g) given that the equilibrium concentrations of carbon monoxide, oxygen and carbon dioxide are 2.0 x 10-6 M, 1.0 x 10-6 M, and 0.25 M respectively.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Calculate K for 2CO2(g) ⇔ 2CO(g) + O2(g)
given that the equilibrium concentrations of carbon monoxi...
Questions



Mathematics, 14.11.2020 14:00

Mathematics, 14.11.2020 14:00

Mathematics, 14.11.2020 14:00


Health, 14.11.2020 14:00



Biology, 14.11.2020 14:00

Mathematics, 14.11.2020 14:00

Advanced Placement (AP), 14.11.2020 14:00




World Languages, 14.11.2020 14:00

English, 14.11.2020 14:00

Computers and Technology, 14.11.2020 14:00

Mathematics, 14.11.2020 14:00

Mathematics, 14.11.2020 14:00