Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
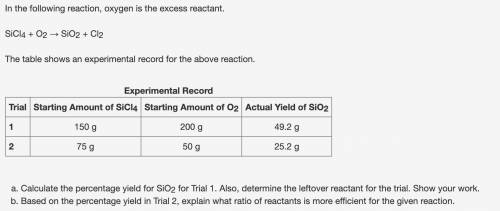
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the t...
Questions


Biology, 31.01.2020 21:44

Mathematics, 31.01.2020 21:44

Biology, 31.01.2020 21:44

Arts, 31.01.2020 21:44


History, 31.01.2020 21:44

Mathematics, 31.01.2020 21:44




Mathematics, 31.01.2020 21:44

Business, 31.01.2020 21:44


English, 31.01.2020 21:44


English, 31.01.2020 21:44



Mathematics, 31.01.2020 21:44