
Chemistry, 21.07.2021 15:10 manuellopez1981
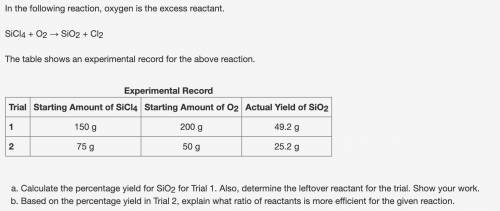
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work. Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the t...
Questions


Mathematics, 21.06.2019 13:00








English, 21.06.2019 13:00









Physics, 21.06.2019 13:00



