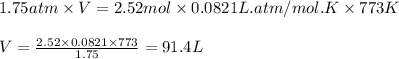Chemistry, 21.07.2021 20:00 imstressed
What volume of water is produced when 38.5 g of ethanol reacts with oxygen at 500°C at 1.75 atm? CH3CH2OH(g) + 3 O2(g)→ 2 CO2(g) + 3 H2O(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

You know the right answer?
What volume of water is produced when 38.5 g of ethanol reacts with oxygen at 500°C at 1.75 atm?
CH...
Questions

Mathematics, 05.11.2020 20:40

Arts, 05.11.2020 20:40



Mathematics, 05.11.2020 20:40

History, 05.11.2020 20:40

English, 05.11.2020 20:40



Computers and Technology, 05.11.2020 20:40

Physics, 05.11.2020 20:40

Mathematics, 05.11.2020 20:40

Mathematics, 05.11.2020 20:40

Arts, 05.11.2020 20:40

Mathematics, 05.11.2020 20:40




English, 05.11.2020 20:40

Mathematics, 05.11.2020 20:40

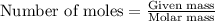 ......(1)
......(1)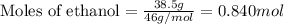
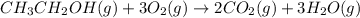
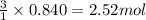 of water
of water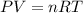 .......(2)
.......(2)![500^oC=[500+273]K=773K](/tpl/images/1397/4323/8da9a.png)