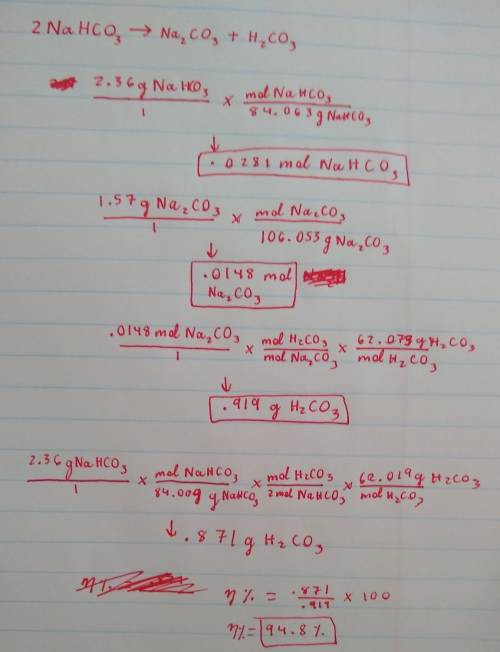ASAP
A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO→ Na...

Chemistry, 24.07.2021 01:00 crookdamian21
ASAP
A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO→ Na2CO3 + H2CO3
In this experiment, carbon dioxide and water vapors combine to form H2CO3. After decomposition, the Na2CO3 had a mass of 1.57 grams.
Determine the mass of the H2CO3 produced.
Calculate the percentage yield of H2CO3 for the reaction. Show your work or describe the calculation process in detail.
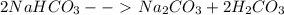

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
Questions




Mathematics, 07.09.2021 04:30

Mathematics, 07.09.2021 04:30


Mathematics, 07.09.2021 04:30


Mathematics, 07.09.2021 04:30

English, 07.09.2021 04:30



Mathematics, 07.09.2021 04:30


Social Studies, 07.09.2021 04:30

Mathematics, 07.09.2021 04:30


Spanish, 07.09.2021 04:30