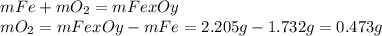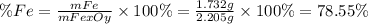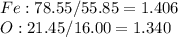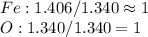Chemistry, 24.07.2021 02:40 briana21092005
A 1.732 g sample of iron is heated in air. It oxidizes to form a product with a mass of 2.205 g. What is the empirical formula of the product?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
A 1.732 g sample of iron is heated in air. It oxidizes to form a product with a mass of 2.205 g.
Wh...
Questions



Biology, 27.03.2020 01:23


Biology, 27.03.2020 01:23

Mathematics, 27.03.2020 01:23


Biology, 27.03.2020 01:23




Mathematics, 27.03.2020 01:23


Mathematics, 27.03.2020 01:23


Mathematics, 27.03.2020 01:23

Mathematics, 27.03.2020 01:23



Physics, 27.03.2020 01:23