
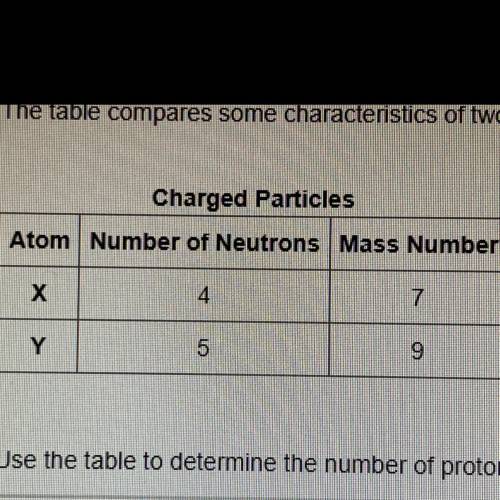
Use the table to determine the number of protons for each atom. Then, choose the statement below that is true about the two atoms.
O Atoms X and Atom Y are in the same family.
O Atom X is in a column to the right of Atom Y on the periodic table.
O Atom X and Atom Y are in the same row on the periodic table.
O Atoms X and Atom Y are isotopes.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Use the table to determine the number of protons for each atom. Then, choose the statement below tha...
Questions



Mathematics, 05.10.2019 02:30

Biology, 05.10.2019 02:30



History, 05.10.2019 02:30


Mathematics, 05.10.2019 02:30



History, 05.10.2019 02:30











