
Chemistry, 25.07.2021 06:20 brooklynunderwood46
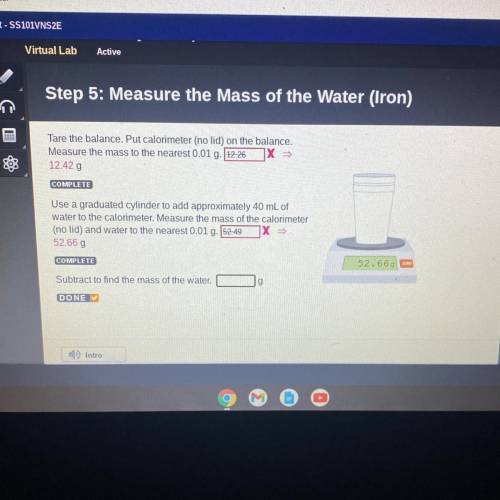
Step 5: Measure the Mass of the Water (Iron)
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g. 12.26 X
12.42 g
COMPLETE
Use a graduated cylinder to add approximately 40 mL of
water to the calorimeter. Measure the mass of the calorimeter
(no lid) and water to the nearest 0.01 g. 52.49
52.66 g
COMPLETE
52.669
Subtract to find the mass of the water.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Step 5: Measure the Mass of the Water (Iron)
Tare the balance. Put calorimeter (no lid) on the bala...
Questions

History, 07.07.2019 21:00

History, 07.07.2019 21:00

History, 07.07.2019 21:00

English, 07.07.2019 21:00

Social Studies, 07.07.2019 21:00




Social Studies, 07.07.2019 21:00




Mathematics, 07.07.2019 21:00



Health, 07.07.2019 21:00


History, 07.07.2019 21:00


Mathematics, 07.07.2019 21:00



