Gu
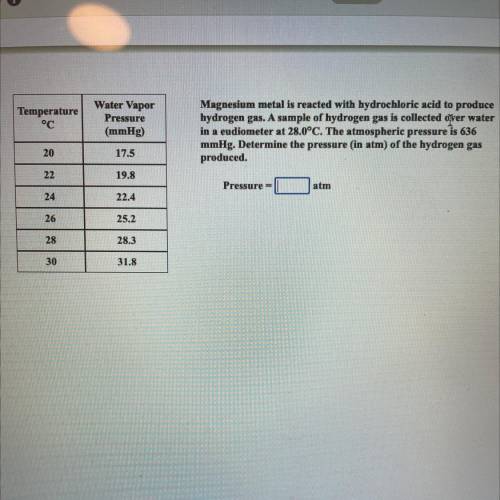
Magnesium metal is reacted with hydrochloric acid to produce
hydrogen gas. A sample of hyd...

Chemistry, 26.07.2021 07:10 natalie2sheffield
Gu
Magnesium metal is reacted with hydrochloric acid to produce
hydrogen gas. A sample of hydrogen gas is collected over water
in a eudiometer at 28.0°C. The atmospheric pressure is 636
mmHg. Determine the pressure (in atm) of the hydrogen gas
produced
Pressure =
atm

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1


Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
Questions

Spanish, 30.06.2019 20:30

Spanish, 30.06.2019 20:30

History, 30.06.2019 20:30





Chemistry, 30.06.2019 20:30



Mathematics, 30.06.2019 20:30


History, 30.06.2019 20:30


English, 30.06.2019 20:30


Mathematics, 30.06.2019 20:30

Geography, 30.06.2019 20:30

History, 30.06.2019 20:30



