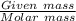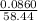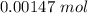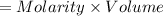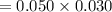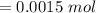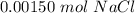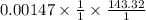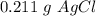Chemistry, 26.07.2021 17:40 matt416760
A sample of 0.0860 g of sodium chloride is added to 30.0 mL of 0.050 M silver nitrate, resulting in the formation of a precipitate. (a) Write the molecular equation for the reaction. (b) What is the limiting reactant in the reaction? (c) How many grams of precipitate potentially form?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3


Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
A sample of 0.0860 g of sodium chloride is added to 30.0 mL of 0.050 M silver nitrate, resulting in...
Questions


Social Studies, 15.02.2021 20:50

Mathematics, 15.02.2021 20:50

English, 15.02.2021 20:50

History, 15.02.2021 20:50


Mathematics, 15.02.2021 20:50

Biology, 15.02.2021 20:50



Medicine, 15.02.2021 21:00


Mathematics, 15.02.2021 21:00

Arts, 15.02.2021 21:00

English, 15.02.2021 21:00

Mathematics, 15.02.2021 21:00



Mathematics, 15.02.2021 21:00

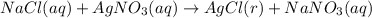
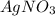 ,
,