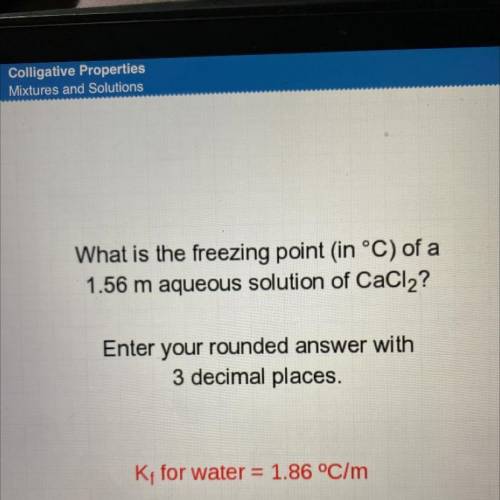
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded a...

Chemistry, 26.07.2021 22:20 joselinegarciaowyrpf
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded answer with
3 decimal places.
K; for water = 1.86 °C/m

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Questions


Physics, 01.09.2020 14:01

Geography, 01.09.2020 14:01

Mathematics, 01.09.2020 14:01

Mathematics, 01.09.2020 14:01

English, 01.09.2020 14:01

English, 01.09.2020 14:01

English, 01.09.2020 14:01





Mathematics, 01.09.2020 14:01


History, 01.09.2020 14:01

Mathematics, 01.09.2020 14:01

Mathematics, 01.09.2020 14:01

Business, 01.09.2020 14:01


Computers and Technology, 01.09.2020 14:01



