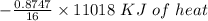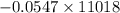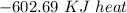Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane calculate the heat associated with the for...
Questions




Health, 05.01.2021 16:30




Business, 05.01.2021 16:30

Biology, 05.01.2021 16:30


Mathematics, 05.01.2021 16:30

Mathematics, 05.01.2021 16:30

English, 05.01.2021 16:30

Biology, 05.01.2021 16:30





Mathematics, 05.01.2021 16:30

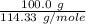
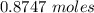
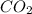 formation associates with -11018 kJ of heat, then
formation associates with -11018 kJ of heat, then