
Chemistry, 28.07.2021 04:40 amcda213040
Suppose that you add 24.3 g of an unknown molecular compound to 0.250 kg of benzene, which has a K f of 5.12 oC/m. With the added solute, you find that there is a freezing point depression of 3.14 oC compared to pure benzene. What is the molar mass (in g/mol) of the unknown compound

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 23.06.2019 09:00
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3

Chemistry, 23.06.2019 10:30
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
You know the right answer?
Suppose that you add 24.3 g of an unknown molecular compound to 0.250 kg of benzene, which has a K f...
Questions

Mathematics, 22.04.2022 14:00

History, 22.04.2022 14:00

Biology, 22.04.2022 14:00

Biology, 22.04.2022 14:00

Mathematics, 22.04.2022 14:00



Mathematics, 22.04.2022 14:00


Mathematics, 22.04.2022 14:00


Mathematics, 22.04.2022 14:00


Mathematics, 22.04.2022 14:00

Biology, 22.04.2022 14:10

History, 22.04.2022 14:10




Mathematics, 22.04.2022 15:30

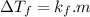 and
and 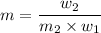
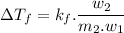 ..................(1)
..................(1)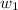 = amount of solvent (in kg)
= amount of solvent (in kg)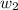 = amount of solute (in kg)
= amount of solute (in kg)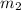 = molar mass of solute (g/mole)
= molar mass of solute (g/mole)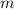 = molality of solution (mole/kg)
= molality of solution (mole/kg) =
= 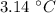 ,
, 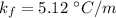
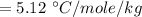
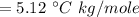
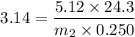
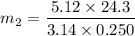
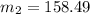 g/mole
g/mole


