
Chemistry, 28.07.2021 15:20 basketball6076
The Ka of hypochlorous acid (HClO) is 3.00*10^-8. What is the pH at 25.0 °C of an aqueous solution that is 0.02M in HClO?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 23.06.2019 10:30
Chemical bonds result from the interaction of the from two or more atoms. a. protons b. electrons c. neutrons d. nuclei
Answers: 2

You know the right answer?
The Ka of hypochlorous acid (HClO) is 3.00*10^-8. What is the pH at 25.0 °C of an aqueous solution t...
Questions

Mathematics, 14.03.2020 02:56





Spanish, 14.03.2020 02:56


Computers and Technology, 14.03.2020 02:56

Biology, 14.03.2020 02:56

Biology, 14.03.2020 02:56





Social Studies, 14.03.2020 02:56

Mathematics, 14.03.2020 02:56




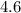 .
.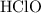 ionizes partially at room temperature:
ionizes partially at room temperature: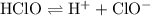 .
.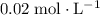 .
.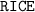 table to analyze the concentration (also in
table to analyze the concentration (also in  ) of the species in this equilibrium.
) of the species in this equilibrium. 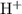 is negligible (around
is negligible (around 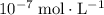 ) when compared to the concentration of
) when compared to the concentration of  be the reduction in the concentration of
be the reduction in the concentration of 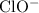 would both increase by
would both increase by 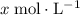 . (
. (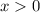 since concentration should be non-negative.)
since concentration should be non-negative.)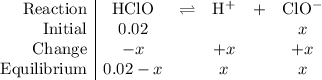 .
.![\rm [H^{+}]](/tpl/images/1401/0171/444a9.png) ,
, ![\rm [ClO^{-}]](/tpl/images/1401/0171/a4200.png) , and
, and ![[{\rm HClO}]](/tpl/images/1401/0171/03e6a.png) denote the concentration of the three species at equilibrium respectively. Equation for the
denote the concentration of the three species at equilibrium respectively. Equation for the  of
of ![\begin{aligned}K_\text{a} &= \frac{\rm [H^{+}] \cdot [ClO^{-}]}{[\rm HClO]}\end{aligned}](/tpl/images/1401/0171/0ead6.png) .
.![\begin{aligned}K_\text{a} &= \frac{\rm [H^{+}] \cdot [ClO^{-}]}{[\rm HClO]} = \frac{x^{2}}{0.02 - x}\end{aligned}](/tpl/images/1401/0171/e139a.png) .
.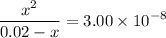 .
. (concentration of
(concentration of 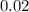 (initial concentration.)
(initial concentration.)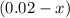 as
as 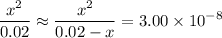 .
. .
.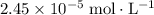 at equilibrium.
at equilibrium.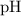 :
:![\begin{aligned}\text{pH} &= \log_{10} ([{\rm H^{+}}]) \\ &\approx \log_{10} (2.45 \times 10^{-5}) \\ &\approx 4.6\end{aligned}](/tpl/images/1401/0171/0fa9b.png) .
.

