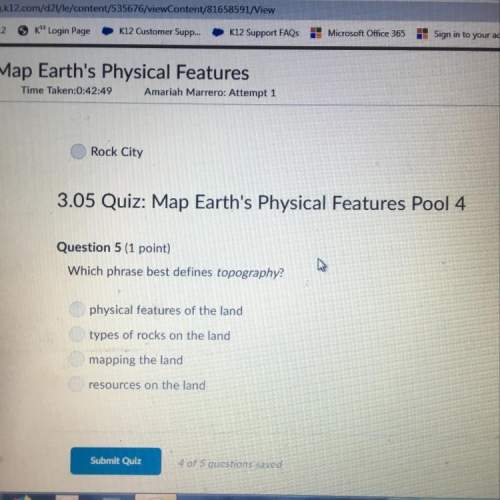
For the decomposition reaction given below: CaCO3 ( CaO(s) + CO2 (g)
Assuming that the initial concentration of CaCO3 is 0.50M and its final concentration after 50minutes of reaction is 0.15M, determine the rate constant and half life for the first order process.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
For the decomposition reaction given below: CaCO3 ( CaO(s) + CO2 (g)
Assuming that the initial conc...
Questions

Mathematics, 20.04.2021 05:50






Mathematics, 20.04.2021 05:50

Mathematics, 20.04.2021 05:50

Mathematics, 20.04.2021 05:50

Biology, 20.04.2021 05:50


Physics, 20.04.2021 05:50

Chemistry, 20.04.2021 05:50

Chemistry, 20.04.2021 05:50


Physics, 20.04.2021 05:50


Mathematics, 20.04.2021 05:50

History, 20.04.2021 05:50

Mathematics, 20.04.2021 05:50