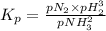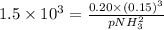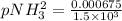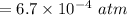Chemistry, 30.07.2021 04:10 CHEVYWADDELL
The decomposition of ammonia is: 2 NH3(g) ⇌ N2(g) + 3 H2(g). If Kp is 1.5 × 103 at 400°C, what is the partial pressure of ammonia at equilibrium when N2 is 0.20 atm and H2 is 0.15 atm?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2


Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
The decomposition of ammonia is: 2 NH3(g) ⇌ N2(g) + 3 H2(g). If Kp is 1.5 × 103 at 400°C, what is th...
Questions


Spanish, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Arts, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00

History, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00



Business, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Arts, 24.11.2020 01:00

History, 24.11.2020 01:00

 " is the right answer.
" is the right answer. ,
,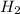 ,
,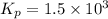 at
at