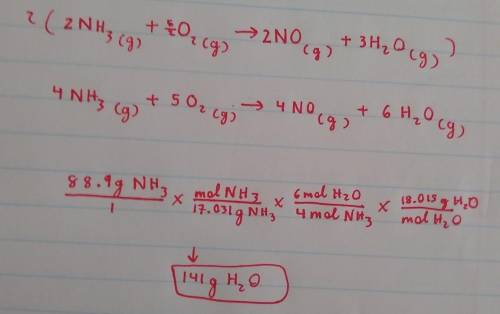Chemistry, 30.07.2021 19:30 cyndalulu6729
If you reacted 88.9 g of ammonia with excess oxygen, what mass of water would you expect to make? You will need to balance the equation first. NH3(g) + O2(g) -> NO(g) + H2O(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Drag each tile to the correct box arrange the layers not order from oldest to youngest
Answers: 2

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2
You know the right answer?
If you reacted 88.9 g of ammonia with excess oxygen, what mass of water would you expect to make? Yo...
Questions

Mathematics, 12.03.2020 22:57





Mathematics, 12.03.2020 22:57

Mathematics, 12.03.2020 22:57


German, 12.03.2020 22:57





Mathematics, 12.03.2020 22:57





Computers and Technology, 12.03.2020 22:57