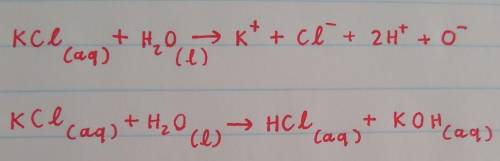When KCl dissolves in water:
the Cl- ions are attracted to the partially negative oxygen atoms of the water molecule.
the K+ ions are attracted to Cl- ions on the KCl crystal.
the K+ ions are attracted to the partially positive hydrogen atoms of the water molecule.
the K+ ions are attracted to the partially negative oxygen atoms of the water molecule.
the Cl- ions are attracted to dissolved K+ ions.
Please explain!

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

You know the right answer?
When KCl dissolves in water:
the Cl- ions are attracted to the partially negative oxygen atoms of t...
Questions


Mathematics, 22.05.2020 22:04

Mathematics, 22.05.2020 22:04

Mathematics, 22.05.2020 22:04

History, 22.05.2020 22:04

History, 22.05.2020 22:04

English, 22.05.2020 22:04


Mathematics, 22.05.2020 22:04

Biology, 22.05.2020 22:04

Mathematics, 22.05.2020 22:04


Spanish, 22.05.2020 22:04

Chemistry, 22.05.2020 22:04


Biology, 22.05.2020 22:05



Biology, 22.05.2020 22:05