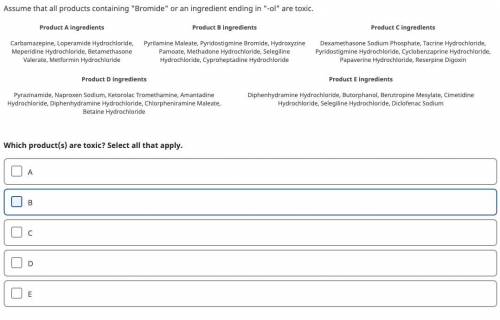
Assume that all products containing “Bromide” or an ingredient ending in “-ol” are toxic.
...

Chemistry, 31.07.2021 14:00 maloynegen7681
Assume that all products containing “Bromide” or an ingredient ending in “-ol” are toxic.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Questions


Health, 25.09.2019 05:30



Mathematics, 25.09.2019 05:30




English, 25.09.2019 05:30

Mathematics, 25.09.2019 05:30




Chemistry, 25.09.2019 05:30

History, 25.09.2019 05:30



Mathematics, 25.09.2019 05:30


Biology, 25.09.2019 05:30



