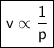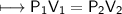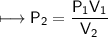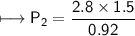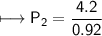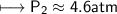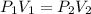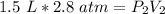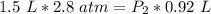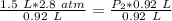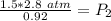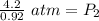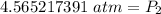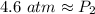Chemistry, 01.08.2021 06:00 emokid7822
At a constant temperature, a sample of gas occupies 1.5 L at a pressure of 2.8 ATM. What will be the pressure of this sample, in atmospheres, if the new volume is 0.92 L?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
At a constant temperature, a sample of gas occupies 1.5 L at a pressure of 2.8 ATM. What will be the...
Questions

History, 14.04.2020 22:29





English, 14.04.2020 22:29






Mathematics, 14.04.2020 22:30


Biology, 14.04.2020 22:30