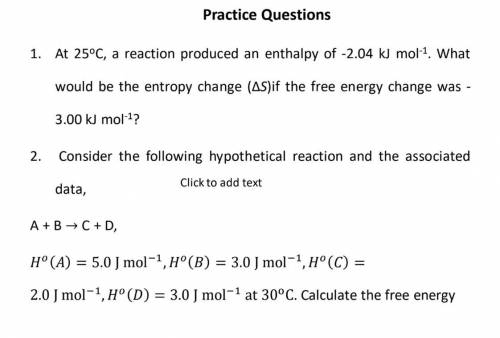
25oC, a reaction produced an enthalpy of -2.04 kJ mol-1
. What
would be the entropy change (Δ...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1
You know the right answer?
Questions

Mathematics, 22.01.2020 19:31



Computers and Technology, 22.01.2020 19:31



History, 22.01.2020 19:31

History, 22.01.2020 19:31



History, 22.01.2020 19:31

English, 22.01.2020 19:31


English, 22.01.2020 19:31



Mathematics, 22.01.2020 19:31